Cell Membranes in Super Resolution
12/02/2020For the first time ever, expansion microscopy allows the imaging of even the finest details of cell membranes. This offers new insights into bacterial and viral infection processes.
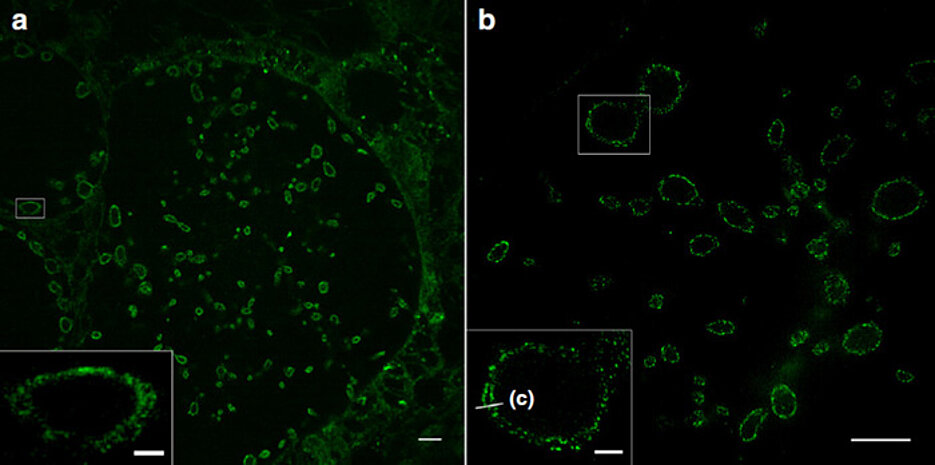
Expansion microscopy (ExM) enables the imaging of cells and their components with a spatial resolution far below 200 nanometres. For this purpose, the proteins of the sample under investigation are cross-linked into a swellable polymer. Once the interactions between the molecules have been destroyed, the samples can be expanded many times over with water. This allows detailed insights into their structures.
"This method was previously limited to proteins. In the journal Nature Communications we are now presenting a way of expanding lipids and thus cell membranes," says Professor Markus Sauer, an expert in super-resolution microscopy from the Biocentre of Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany. JMU professors Thomas Rudel (microbiology) and Jürgen Seibel (chemistry) are also involved in the publication.
Synthetic lipids are marked and expanded
Jürgen Seibel's team has synthesised functionalised sphingolipids, which are an important component of cell membranes. If these lipids are added to cell cultures, they are incorporated into the cell membranes. They can then be marked with a dye and expanded four to ten times in a swellable polymer.
The JMU researchers show that this method – in combination with structured illumination microscopy (SIM) – makes it possible for the first time to image different membranes and their interactions with proteins with a resolution of 10 to 20 nanometres: cell membranes, the outer and inner cell nuclear membrane and also the membranes of intracellular organelles such as mitochondria.
Focusing on bacteria and viruses
The sphingolipids also integrate highly efficiently into the membranes of bacteria. This means that, for the first time, pathogens such as Neisseria gonorrhoeae, Chlamydia trachomatis and Simkania negevensis can now be visualised in infected cells with a resolution that was previously only achieved using electron microscopy. Even the inner and outer membranes of Gram-negative bacteria can be distinguished from each other.
"With the new super-resolution microscopic methods, we now want to investigate bacterial infection mechanisms and causes of antibiotic resistance. What we learn in the process could possibly be used for improved therapies," says Professor Thomas Rudel, an expert on bacterial infections.
The sphingolipids might also integrate into the membrane of viruses. If this is successful, the interactions of corona viruses with cells could be studied for the first time with high resolution light microscopy.
The work described was financially supported by the German Research Foundation (DFG) within the framework of Research Training Groups 2157 and 2581.
Publication
Nanoscale imaging of bacterial infections by sphingolipid expansion microscopy. Nature Communications, 2 December 2020, DOI: 10.1038/s41467-020-19897-1
Contact
Prof. Dr. Thomas Rudel, Chair of Microbiology, University of Würzburg,
T +49 931 31-84401, thomas.rudel@biozentrum.uni-wuerzburg.de
Prof. Dr Markus Sauer, Chair of Biotechnology and Biophysics, University of Würzburg,
T +49 931 31-88687, m.sauer@uni-wuerzburg.de