Flash mob in the nucleus
06/22/2021Some proteins concentrate in certain places in the cell nucleus. A new study with Würzburg participation now shows how this happens. The results could contribute to a better understanding of a rare disease.
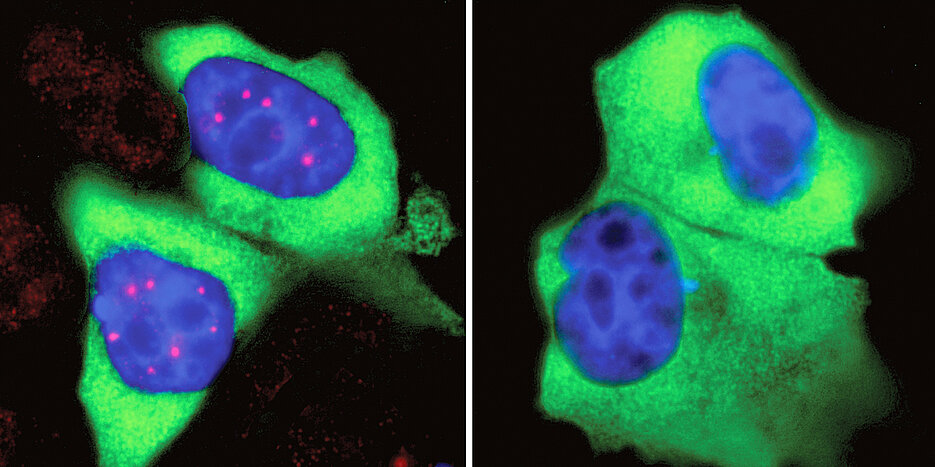
Almost all cells in our body contain a nucleus: a somewhat spherical structure that is separated from the rest of the cell by a membrane. Each nucleus contains all the genetic information of the human being. So it serves as a kind of library - but one with strict requirements: If the cell needs the building instructions for a protein, it won’t simply borrow the original information. Instead, a transcript of it is made in the nucleus.
The machinery required for this is very complex, not least because the transcripts are not simple copies. In addition to essential information, genes also contain numerous passages of meaningless "garbage". They are removed when the transcript is made. Biologists call this editorial revision "splicing".
A molecular architect and controller
"An important role in splicing is played by the SMN complex, a 'molecular machine' consisting of nine different proteins," explains Prof. Dr. Oliver Gruss from the Institute of Genetics at the University of Bonn. The SMN complex mediates an essential function for cells by assisting in the assembly of other macromolecular structures. "It thus fulfils the task of a molecular architect and controller, without which certain functions in our cells would be unthinkable," adds Professor Utz Fischer.
Fischer holds the Chair of Biochemistry at the Julius Maximilian University of Würzburg (JM)U). Together with his colleague Dr. Archana Prusty and researchers from the Universities of Heidelberg and Bonn and with the help of Evotec SE at the Martinsried site, he has gained new insights into the processes in the cell nucleus. In the current issue of the journal Cell Reports, the team presents the results of their work.
For the SMN complex to perform its function correctly, it must be precisely coordinated both spatially and temporally. Therefore, its dynamics in the cell were of great interest to the researchers. "Interestingly, the SMN complex is not evenly distributed in the nucleus. Instead, it accumulates at specific sites called Cajal bodies," Fischer explains. However, there are no transport mechanisms in the cell nucleus that bring the SMN complexes to the Cajal bodies. Instead, the SMN complex itself has certain properties that are responsible for its "rooting together". What these are was unclear until now.
SMN complexes carry an unusually large number of phosphate groups
SMN complexes have a prominent feature: They carry an unusually large number of phosphate groups, which are small molecular residues with a phosphorus atom in the center. "We suspected that this phosphorylation promotes their mass clustering into Cajal bodies," explains Dr. Maximilian Schilling from the research group around Prof. Gruss.
Phosphate groups are not part of the actual blueprint of a protein - they are added later and can also be removed again. This is often how the cell regulates the activity of the respective protein. The phosphate group is attached in this process by certain enzymes, the kinases. "We have now inhibited each of the hundreds of human kinases individually and looked at how that affects the formation of Cajal bodies," Schilling says.
In this way, they encountered a network of kinases, which, when inhibited, caused the Cajal bodies to largely disappear. Further analyses showed that in the absence of these kinases, phosphorylation of SMN complexes at specific sites decreased sharply. This then causes the flash mobs in the nucleus to cease - the Cajal bodies disintegrate. The finding is particularly interesting because the kinases identified not only regulate splicing, but also the translation of the gene transcripts edited in this way into proteins. These are therefore enzymes that are crucial for various steps in this vital process.
Mutation causes severe disease
The SMN complex is not only known for its role in splicing: "If mutations lead to a reduced formation of the SMN protein, the eponymous component of the SMN complex, this leads to a severe disease in those affected, spinal muscular atrophy," explains Utz Fischer, who has been researching the causes of this disease for many years. One in about 6,000 newborns is born with this genetic defect.
Treatment is extremely expensive; the cost per patient runs into millions. "Some of the gene defects that cause spinal muscular atrophy are near the phosphorylation sites of the SMN complex," explains Gruss. "Affected individuals may therefore have impaired attachment of phosphate groups to these sites, and consequently also impaired formation of Cajal bodies. We suspect that this causes splicing to be impaired, which subsequently results in the disease symptoms."
The kinases identified may therefore also be suitable as a starting point for new therapies. Preliminary results from mouse model cells for human spinal muscular atrophy show that agents that increase kinase activity also improve Cajal body formation. "It is completely unclear whether these agents also ameliorate pathological changes in a complex organism," cautions Gruss against inflated expectations. "That new treatment options will eventually emerge from this is therefore still speculation at this stage."
Participating institutions and funding
The universities of Bonn, Würzburg and Heidelberg were involved in the study. It was funded by the German Research Foundation (DFG) and the US-American CURE SMA Foundation.
Publication
Maximilian Schilling, Archana B. Prusty, Björn Boysen, Felix S. Oppermann, Yannick L. Riedel, Alma Husedzinovic, Homa Rasouli, Angelika König, Pradhipa Ramanathan, Jürgen Reymann, Holger Erfle, Henrik Daub, Utz Fischer and Oliver J. Gruss: TOR signaling regulates liquid phase separation of the SMN complex governing snRNP biogenesis; Cell Reports; DOI: 10.1016/j.celrep.2021.109277
Contact
Prof. Dr. Utz Fischer Utz, Lehrstuhl für Biochemie, T: +49 931 31-84029, utz.fischer@biozentrum.uni-wuerzburg.de